Silence descends on the operating theatre and the tension builds until it’s almost a physical presence in the room.
Surgeons have just connected a pig’s kidney to a human body. The clamps have been released and human blood is now flowing into the pig organ.
“You could have heard a pin drop,” says transplant surgeon Dr Jayme Locke.
Success or failure will be determined in moments and there is now just a single question on everyone’s minds: “Pink or black?”
Using animal organs in the human body is an old idea, and has ranged from “zest for life” chimpanzee testicle implants to replacement kidneys and hearts taken from our primate relatives. The latter often ended in death soon afterwards. The problem is, our immune system treats the transplanted organ like an infection and attacks.
The focus these days is on pigs, as their organs are roughly the right size and we have centuries of experience farming them.
But the challenge of hyperacute rejection – keeping organs pink, not black – is the same. You can’t just pop down to the farm, choose a pig and transplant its organs. It’s taken huge advances in genetic engineering to alter pigs’ DNA so their organs are more compatible with our immune systems.
If the body unleashes a horrendous assault on the foreign organ – holes will be ripped in every cell in the pig tissue and the organ will clot from the inside out. It will go splotchy, then blue, then completely black within minutes.
If “hyperacute rejection” is avoided, the organ will blush pink with the flow of blood and oxygen.
“It turned beautiful and pink… the sense of relief, the sense of joy and hope just filled the room. We might have high-fived as well,” said Dr Locke, from the University of Alabama at Birmingham, in the US.
This operation was just one of a series of medical breakthroughs that have renewed interest in the field of xenotransplantation.
The recent kidney and heart transplants have taken organs from the specially designed “10-gene pig”.
It has one genetic tweak to prevent any donated organs responding to human growth hormones and growing out of control.
Another key alteration removes a sugary molecule, called alpha-Gal, which sticks to the surface of pig cells and acts like a gigantic flashing neon sign marking the tissue as absolutely alien.
A wing of our immune system, called the complement system, is constantly patrolling the body looking for alpha-Gal. That’s why organs can be rejected and killed moments after they are transplanted.
Two other “neon signs” were genetically removed and six human ones added in, acting like a camouflage net over the pig cells to help hide them from the immune system.
The resulting 10-gene pigs are then raised in sterile conditions so they are suitable for transplant.
The pair of pig kidneys were transplanted into the brain-dead body of Jim Parsons in September 2021.
He’d wanted to be an organ donor when he died and when his kidneys were donated, pig kidneys were put in their place with permission from his family.
Dr Locke describes the moment one of the kidneys started making urine as “remarkable” and feels xenotransplantation can “really change people’s lives, and to be blunt, save their lives”. She’s expecting to start clinical trials later this year.
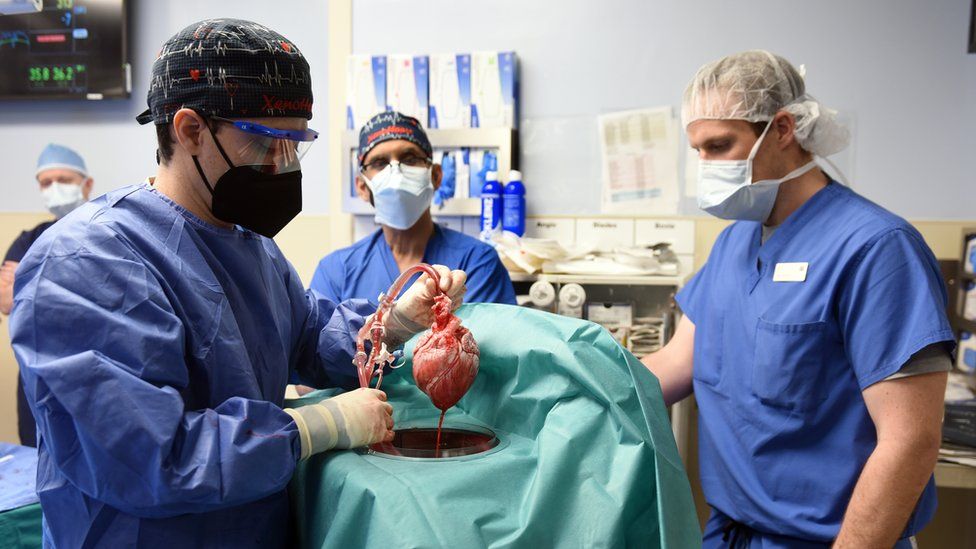
That operation was a three-day-long experiment, but meanwhile, surgeons at the University of Maryland Medical Center were about to go one step further.
Their patient, David Bennett, 57, had severe heart failure. He was not deemed suitable for a human heart transplant and was being kept alive by an Ecmo machine, which supported his heart and lungs.
Mr Bennett described having a pig heart as a “shot in the dark”.
A 10-gene pig was driven to the hospital and on 7 January, its heart was placed inside David Bennett’s chest. The operation was tricky because Mr Bennett’s diseased heart had swollen, so connecting the blood vessels to the smaller pig-heart was a challenge.
Again there was the nervous moment to see whether the heart would be rapidly rejected, but it was beating and stayed pink. Dr Muhammad Mohiuddin, the hospital’s director of cardiac xenotransplantation, said he did not expect to witness this “in my lifetime”.
When I spoke to him on the one-month anniversary of the operation he said there were no signs of the organ being rejected, but Mr Bennett was still frail.
“We put a new Ferrari engine in a 1960s car. The engine is working great but the rest of the body has to adjust,” he said.
But Mr Bennett died two months after the transplant. The cause, and therefore the implications for xenotransplantation, is still uncertain.
Mr Bennett was very frail before the operation and it is possible that even the new heart was not enough.
No signs of organ rejection have been reported, but if detailed analysis of the heart shows signs of the immune system attacking it, the 10-gene pig may need further modifications to make its organs suitable for the human body.
Alternatively, it could come down to anatomy, and pig hearts may not be up to the job in a human body. Our hearts have to work much harder to fight gravity than a pig’s because we walk on two legs rather than four.
Chris Denning, a professor of stem cell biology at the University of Nottingham, said overcoming hyperacute rejection meant the heart transplant would be considered “a success”. He said if the issue was frailty, then xenotransplantation “could be successful in the future” but if it came down to anatomy then it could “be potentially a show-stopper”.
The hospital plans to continue doing clinical trials.
Pig hearts do not have to be as good as a human heart to still save huge numbers of lives, according to Prof John Wallwork, one of the UK’s most eminent transplant surgeons. Many people die waiting for a transplant.
Prof Wallwork, who conducted the world’s first heart-lung-liver transplant and was an early pioneer of xenotransplantation, says it’s better to give 1,000 people a 70% chance of survival with a pig heart than 100 people an 85% chance with a human heart.
“So if it’s not quite as good as human transplantation, then we’ve still done more good than not doing the 1,000 patients,” he said.
Xenotransplantation has always felt like the next big thing in transplant medicine. There’s no doubt a series of landmark operations have been performed, but only more research will tell us whether the field – and its grand dreams – will ever come of age.
Dr Locke added: “Our goal would be to have one 10-gene edited pig be able to save a patient with kidney failure, a patient with liver failure, a patient with heart failure and a patient with end-stage lung disease.
“That would be a remarkable accomplishment and I genuinely believe that we will be there during my lifetime.”






